Niramai receives US FDA clearance for medical device SMILE-100 System
The SMILE-100 System assists experts to visualise high thermal activity patterns clearly demarcated on thermal images as hotspots and can enable healthcare professionals to make better decisions in breast cancer screening and diagnosis.
Niramai Health Analytix, the deep-tech Bengaluru-based healthcare company offering a novel radiation-free, non-touch, accurate, breast cancer screening solution in India, has now received US FDA clearance for their first device, SMILE-100 System.
The breast thermography device assists healthcare personnel to review, measure and analyse thermally significant indications in the breast region. It can be used in the hospital, acute care settings, outpatient surgery, healthcare practitioner facilities or an environment where patient care is provided by qualified healthcare personnel.
The SMILE-100 System, according to a statement, helps experts to visualise high thermal activity patterns clearly demarcated on thermal images as hotspots and can enable healthcare professionals to make better decisions in breast cancer screening and diagnosis. SMILE-100 also uses patented AI-based algorithms to check the quality of input thermal images, which can reduce the errors in thermal image capture and enable low skilled health workers to confidently perform the imaging.
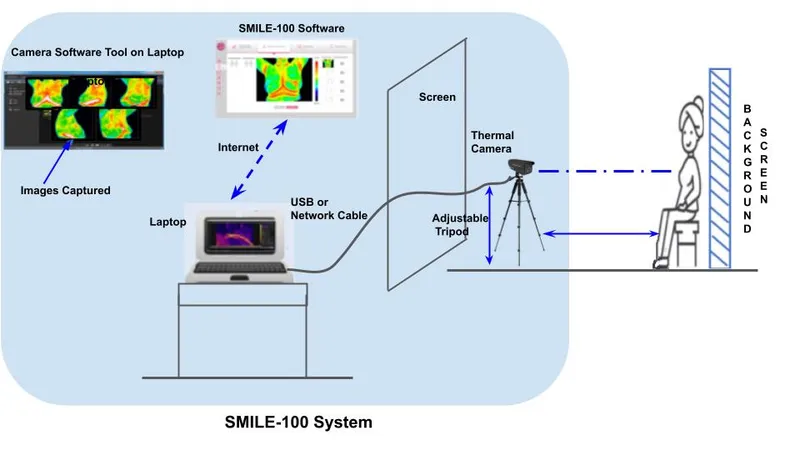
Niramai's SMILE-100 System
Dr. Geetha Manjunath, CEO and Founder of , said, “We are super excited to announce the FDA 510(k) clearance of SMILE-100 System. A proud moment for the team. It was a great learning experience to go through the very rigorous US regulatory process. ”
NIRAMAI was co-founded in July 2016 by Dr. Geetha Manjunath and Ms Nidhi Mathur. It has so far raised a total of $7 million from institutional investors from India, Japan and Singapore. Investors include Dream Incubator, BeeNext, pi Ventures, Ankur Capital, Axilor Ventures, 500 Startups, and others. Last year, the company announced European CE approval for their Thermalytix™ Solution and have already started pilots in multiple European countries. This FDA approval will now give Niramai a tool to foray into the US market and beyond.
Mr. Manish Singhal, Founding Partner of pi Ventures and the lead investor of Niramai who has been part of their journey since 2017, commented, “In the last five years, we have seen Niramai grow in every aspect – product innovation, team strength, clinical provenance, product adoption and international recognition. I congratulate the team for this exciting win of the FDA clearance for their SMILE-100 System. It’s a great feat for any Indian Startup to get US approval for their medical device. This can be the key inflection point for the company to take their product truly global, saving lives all over the world.”



![[Product Roadmap] From breast cancer to river blindness, Niramai is using tech to simplify detection](https://images.yourstory.com/cs/2/a9efa9c02dd911e9adc52d913c55075e/800x400-1588662726076.png?fm=png&auto=format&h=100&w=100&crop=entropy&fit=crop)




