How to ensure the quality and usability of medical devices
In the absence of strict laws regulating the quality of healthcare devices, it is extremely important to invest in a device that guarantees accuracy/safety/consistency under all conditions.

One of the biggest aspirations of every country is to be able to provide QUALITY healthcare to its citizens. This is a difficult task for developing countries such as India where the administration faces numerous challenges that includes reaching to remote parts of the nation, keeping quality standards high while keeping it affordable to the masses. The central and state governments in India have done a commendable job in some sectors. In just about 70 years, the administration has worked tirelessly to get rid of debilitating diseases such as Polio, Leprosy, Smallpox, and so on. Today, India is striving to reach the next destination; that is to be the "medical tourism destination" — where world-class health care is provided at fraction of the cost. Currently, the healthcare industry is estimated to be around 800 million business. This explosive growth has tremendous advantages for our economy but has some significant downside too.
To appreciate the good work, and to understand the drawbacks of the current scenario in India, let us first understand the pillars of a quality healthcare system. The quality of any healthcare delivery system is governed by the quality of three interdependent Ds: Doctors, Drugs, and Devices. The combined quality of entire value chain is jeopardised if any of these Ds fails or if it is sub-optimal.
The Indian government is trying to ensure crème layer of Doctors by providing quality education and training that are regulated by Medical Council of India (MCI). MCI tightly controls the entire medical education system in India to make it uniform as well as of the best quality. The result is that today India produces some of the finest doctors in the world.
The second 'D' of the D-trilogy are the Drugs. Again, the government agency FDA India/CDSCO is doing an amazing job to tightly monitor and control quality of drugs sold to people. It is wonderfully improving the quality of drugs while keeping the prices under control. There is a conscious effort to try and be at par with world standards
Now coming to the third D of the puzzle, i.e. ‘Devices’, here there is a huge gap and there is lots to be desired/achieved. Government is trying to support and bring up devices industry under the umbrella of Innovation/Startup Funding/Made in India campaigns, which is helping companies to pick up business, showcase devices and at the same time provide employment opportunities. On the other hand, the lack of stringent quality standards gives an opportunity for device manufacturers to sell their products in open market without any restriction.
At present, other than a clinical thermometer and X-ray machine, there are no mandatory compliance standards for any other medical device. While some basic standards do exist for some types of devices, they are made ‘voluntary’ in nature and therefore completely and safely ignored by medical device manufacturers of India. Besides, another drawback of this is that sub-standard medical devices manufactured all over the world can be “dumped” in the Indian market – devices and equipment that fail to sell in other parts of the world can be easily sold in Indian market; the only thing that can stop them is some custom-related formalities or checks.
This is ironical and highlights stark contrasts in government policies and the importance given to the quality of medical devices. For example, the sale of mobile phones without CE certification is not allowed, but electronic medical devices such as handheld ECG monitors, electronic BP machines, electronic stethoscope, etc., are sold without CE certifications, or even without being ROHS compliant. ISI mark is mandatory for electrical devices, but BSI marking is not mandatory for medical devices. Even in the construction industry, if a building collapses due to poor quality of material, the responsible builders or contractors are placed under trial and punished. However, nobody would notice if a patient dies or his/her condition worsens due to a wrong dosage of insulin injected with the wrong reading of the Glucometer. There are absolutely no laws that would hold anyone accountable for this.
In this digital era, almost 98 percent of the Analogy medical devices have been replaced by Digital Equipment, which means 98 percent of the medical devices have some sort of electronics inside them. Any kind of electronics is prone to variations in outputs generated due to environmental conditions.
One electronic device or an electrical signal can interfere with another device. This interference can cause damage varying from a small difference in outputs to huge performance impacts. There are also examples of many such medical devices causing painful death or permanent damages. This is the reason why mobiles are switched off in flights, sometimes patients with pacemakers/defibrillators or pregnant women are prohibited from getting X-ray done or asked to not use laptops.
If proper design guidelines related to Electromagnetic Interference, Electromagnetic radiations, Electrostatic discharge, use of hazardous materials and numerous others are not followed, the device can not only give wrong readings, but can also cause health hazards. An extremely talented doctor using an unreliable/inconsistent/low quality device for diagnosis or treatment is equivalent to an F1 racer being asked to drive an Indian truck. Basically, if a device fails, the doctor will not be able to diagnose correctly or may not be able to diagnose at all. To conclude, if the quality of third ‘D-Device’ is not addressed, the entire healthcare system or value chain will be jeopardised.
Medical devices can be broadly categorised in two sections:
Category 1 - Devices that can be operated only by trained professionals. For example, CT Scan machine, Ultrasound machines, MRI Scan machines, etc.
Category 2 - Devices that can be operated by an untrained person. For example, handheld ECG machines, BP monitors etc.
Both categories mentioned above are prone to variations due to electromagnetic field; however, Category 1 is less prone to errors because it is usually operated under controlled environments. Such equipment is installed in hospitals by trained professionals, in an environment that is away from High Voltage Electric lines or away from areas that are prone to electromagnetic interference. Almost all Category 1 devices are imported from countries which follow stringent laws to qualify a medical device. This offers assurance that the device will not give an erroneous reading at extreme conditions.
Category 2 devices are the ones mostly used by the common man on a daily basis and can be purchased from open market easily. They are more prone to such variations because they are being used by patients or attendees anywhere and everywhere without analysing the surrounding conditions for usage.
The impact of interference on devices such as pacemakers, installed inside human body is much higher. Close to 405 EMI problems were suspected by FDA between 1995 and 2005, with 6 deaths, 170 injuries and 167 malfunctions.
The Figure 1 below shows impact of power lines on a ECG waveform captured:
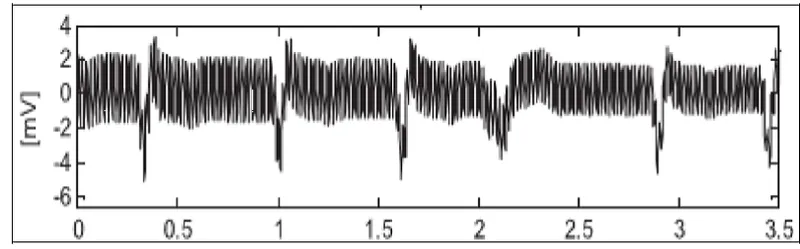
The Figure 2 below shows variations in electrode-skin impedance and activities such as patient’s movements and breath, causing baseline wander in ECG signal.
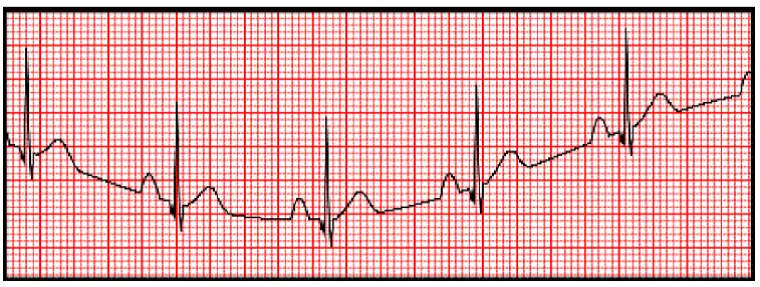
The Figure 3 below shows impact of an electrical appliance on ECG.
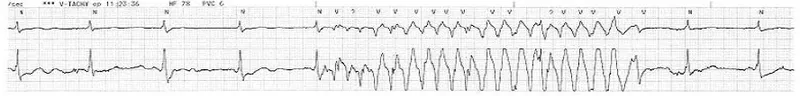
The government should try to balance costs, foster innovation while ensuring that devices are at least meet acceptable levels of quality. However, in this race against time, things do fall between the cracks. So, what is the solution? And how can we, the common people or users, ensure that we don't end up using sub-standard medical devices?
Before buying medical equipment used for diagnostic/monitoring purpose for your clinic or home, you should analyse certain aspects, especially if you are planning to use that device at your home. This is crucial because even mobile phone radiations can easily interfere with your electronic medical device, or the device can give different readings at different battery levels. Until the government comes up with stringent laws for such devices, we have to fend for ourselves, because in case of causalities, it is difficult to get legal support.
Following are the major points to be considered when purchasing a medical device.
Checks for usability:
1) Understand the purpose and usage. For periodic check-up, small device may be fine. For chronic illness, continuous/constant monitoring is needed; hence, more memory storage is needed
2) List down the required features. If it’s an ultrasound machine, then is it 2D or 3D or 4D?
3) If it's a battery-operated device, check if it is rechargeable or the batteries need replacement. If it works on replaceable batteries, then are those batteries available readily?
4) If it is battery powered then what is the battery life, and are there indicators for battery level?
5) If it is an AC powered device, check the power socket specifications, e.g., current, voltage, pin type.
6) Check if there is any specific sterilisation procedure required for any part of the equipment.
7) In case some features of the device work on wireless (Wi-Fi, GPRS/3G/4G, Bluetooth, etc.), check if it is compatible with other devices that you own such as the Wi-Fi router, mobile phone, etc.
8) Go through the different models available and select the best suited option, e.g., portable/handheld/wrist worn/table top/wall mount.
9) If it is a probed device, check if size of probe fits for your intended body part, e.g., SPO2 meter clips should fit your finger size.
10) Check the weight of device based on how portable it should be.
11) Check if you need any visual or audio alarm for an emergency.
12) If it is a device plugged to mobile, check if you need Android or IOS.
13) Check if it needs calibration, frequency of calibration, plus the location of the calibration/service centre.
14) Check after-sales support, the location of the service centre, warranty, and return policy
Checks for quality:
1) Check the quality certification that needs to be fulfilled by specific devices all around world. Handheld devices have a set of medical standards to be fulfilled.
2) Get details of general quality standards fulfilment for this category of medical devices. ECG has Electrocardiogram related standards to be fulfilled, Electronic Home Blood pressure monitor has AAMI and BHS standards for accuracy.
3) Check if the device is certified by any accreditation body, e.g., FDA approval.
4) Check if the device can be used for the specific functionality in the conditions you plan to use it.
5) Check the accuracy and resolution of a device; do compare it with similar products.
6) If there are displays on device, check the pixel strength and graphics clarity.
7) In case the device works by being in contact with body, then check the material used for those contact points from the point of view of impact of dirt/sweat, tendency to corrode, etc.
To conclude, it is extremely important to invest in a device that guarantees accuracy/safety/consistency under all conditions. The best thing is to get a controlled device that has passed Reliability/EMI/EMC/ Electrical/Mechanical/Safety tests, ensuring that it gives correct reading. If the testing quality is rigid, the medical device cannot pass these tests unless it has been designed properly. In absence of strict laws, it is imperative that the user must take utmost measures to choose the right equipment for them. Any kind of investment for medical equipment is for long-term use; hence, necessary care should be taken during the purchase of device.
(Disclaimer: The views and opinions expressed in this article are those of the author and do not necessarily reflect the views of YourStory.)







