How Coeo Labs, through VAPcare, is helping both doctors and patients fight coronavirus
In conversation with Shradha Sharma, Founder and CEO, YourStory, Nitesh Kumar Jangir, Co-founder, Coeo Labs, explains how their product VAPcare protects doctors from ventilated coronavirus patients.
Many Indian startups and entrepreneurs are doing their best to develop the healthcare infrastructure to fight the coronavirus crisis. With the rising number of coronavirus positive cases, some startups are going the extra mile to help solve the problem.
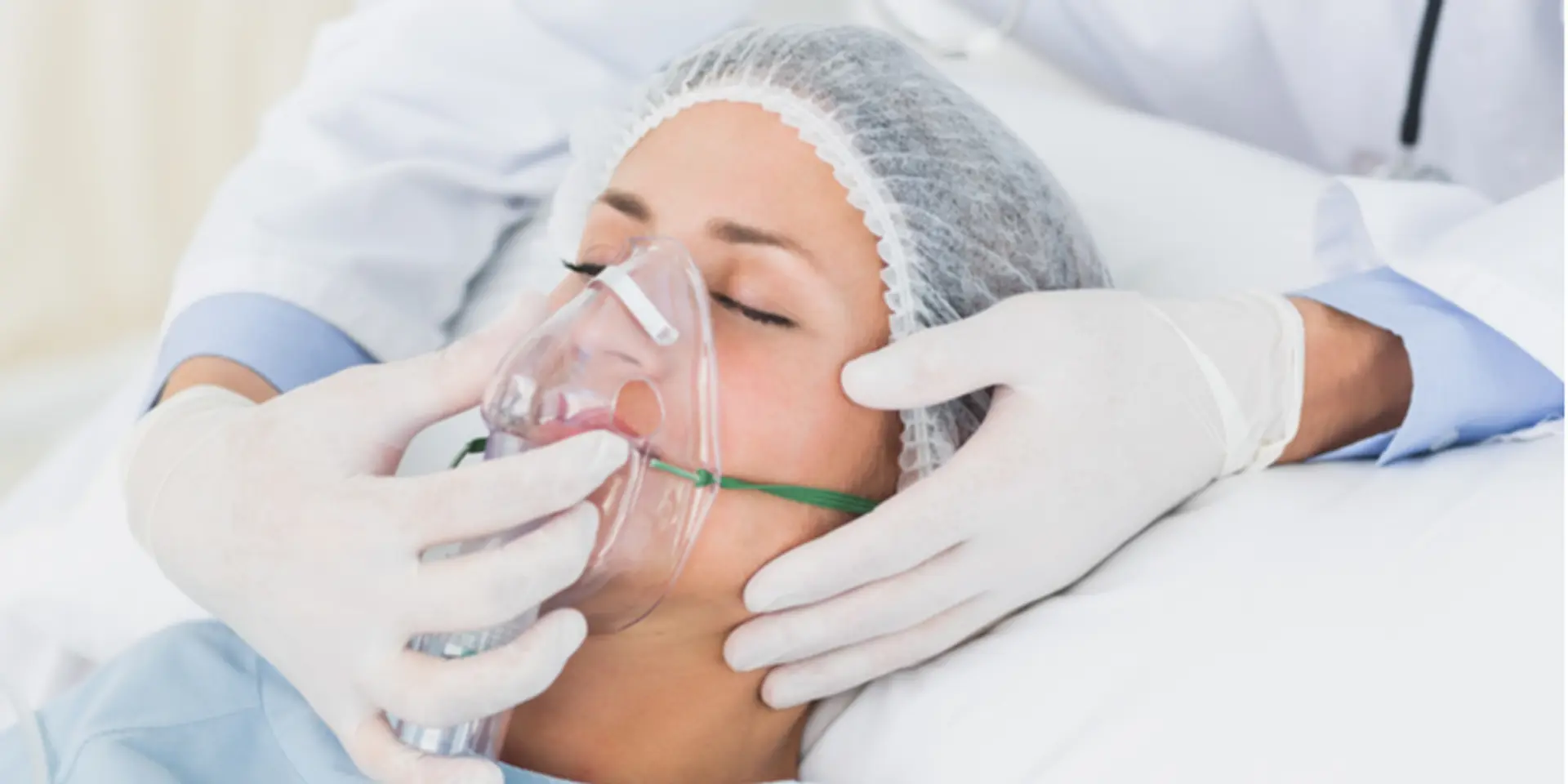
Bengaluru-based emergency and critical care company is helping fight the pandemic by trying to keep the doctors and healthcare professionals safe from getting infected by patients. Coronavirus causes a respiratory disease which can spread to other people when they come in contact with the respiratory droplets of the affected person.
Healthcare providers who need to suction saliva and secretions from ventilated coronavirus patients are at risk of contracting the virus. Coeo Labs’ product VAPcare is keeping the doctors safe as it carries out the entire saliva suctioning process automatically without human intervention.
How does it work?
Speaking with Shradha Sharma, Founder and CEO, YourStory, Coeo Labs Co-founder Nitesh Kumar Jangir, explains, “VAPcare is an intelligent secretions and oral hygiene management system. This is a system which goes inside the patient’s airway, especially those patients who are on ventilators, and automatically senses and removes all the infected saliva and secretions without human intervention to make sure that the patient does not get any secondary infection.”
VAPcare is a sensor-based device used to manage secretions and oral hygiene in the patient’s oral cavity, the oropharyngeal, and the subglottic areas.
Nitesh says this machine is becoming more and more crucial not only because it prevents secondary infection in the patient, but the automatic secretion removal procedure ensures that doctors and nurses do not come in contact with the virus.
“WHO has already declared that open suctioning of the saliva and secretion is one of the ways for transmission of coronavirus and we are putting our doctors and nurses at risk by doing this process manually because right now they don’t have any other option. Our machine can eliminate this risk by doing this automatically and so it can save both patients, as well as caregivers, who are dealing with them,” Nitesh adds.
According to Nitesh, the VAPcare products have been currently installed in some hospitals, coronavirus specific centres, and isolation wards in Andhra Pradesh. “We are getting requests from multiple hospitals who are putting in their efforts in tackling coronavirus in their own states. We look forward to those requests and are trying to figure out how we can manufacture more machines to serve them.”
Apart from this, the company has also upgraded its second product called Saans, a neonatal CPAP machine, which acts as a breathing support machine for babies with respiratory problems. In a bid to combat the coronavirus crisis, the company has upgraded the machine by changing the amount of air delivered through the machine to help coronavirus affected patients.
“The product is ready for deployment and as per our calculation it can help save at least 20 to 30 percent of the patients who need breathing support, and reduce the load on actual ventilators,” Nitesh said.
Deployment of the machines
Recently, Coeo Labs was nominated by Bengaluru-based incubator Centre for Cellular and Molecular Platforms (C-CAMP) as deployment-ready COVID-19 innovation.
Nitesh said that currently the machines have not been used on any COVID-19 patients in India as fortunately not many patients have reached a point where they need ventilators.
“However, we received requests from the US and some countries in Europe and have already shipped some of the devices to these countries where patients are being ventilated. “By next week we will have the products being used on COVID patients over there. The product was designed to treat respiratory diseases and situations arising out of coronavirus crisis,” Nitesh added.
While the company has all the required regulatory approvals for deployment and shipping of the products, it has been facing trouble in getting logistics or courier services to ship the products to long distances.
Edited by Javed Gaihlot