
Addressing Medical Device Startups Challenges With ERP
India is amongst the top 20 medical device industry in the world and this industry functions with accuracy and dedication here. To know more, read here.
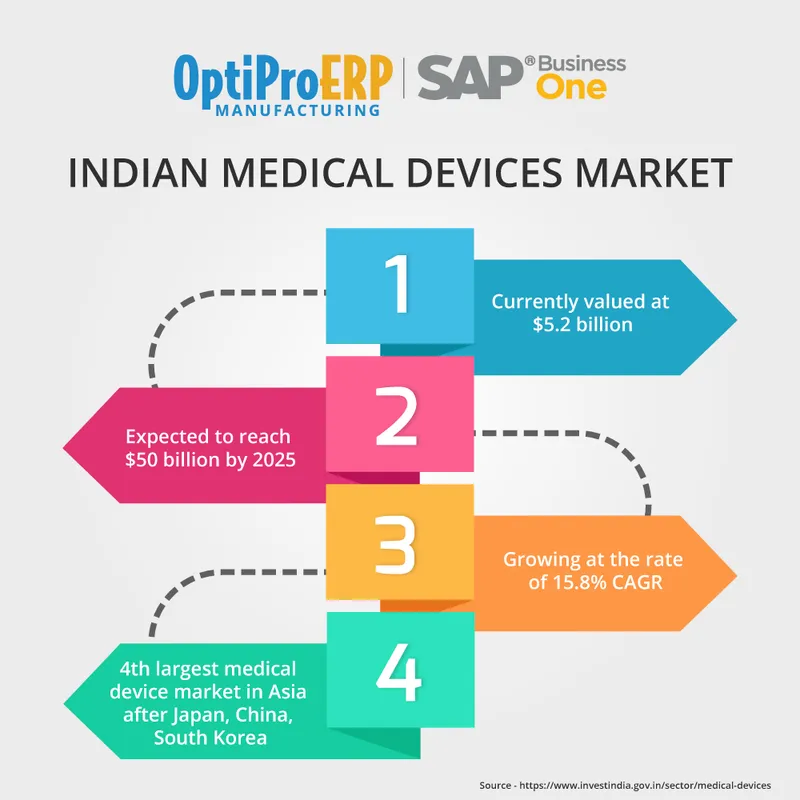
“Indian medical devices market is currently valued at around $5.2 billion and is expected to reach $50 billion by 2025”
Indian medical device industry is a sunrise industry, which focuses on multiple segments such as consumables and disposables, implants, stents and more.
Ranging from artificial joints to surgical equipment and medical consumable products, medical device manufacturers work on an array of products, which are listed below in this blog.
Talking about Indian geography, this market is growing at the rate of 15.8% CAGR and is among the top 20 global medical devices market. Also, it is the 4th largest market in the medical device sector after Japan, China and South Korea.
These are the major segments in Indian medical device –
- Consumables and disposables: staples, needles, gowns, adhesive, masks, tubing, syringes, catheters, sutures, medical gloves etc.
- Diagnostic imaging: it includes MRI scan, X-rays, CT scans, ultrasound. Different devices and techniques let the practitioner produce pictures of the activities done in your body.
- Orthopedic products: with the provision and use of artificial devices such as splints and braces, and artificial limbs (prostheses, medical professionals aim to increase the functions and lifestyle of the patient.
- Dental products: floss, waxes, excavators, operative burs and more.
- Equipment and instruments: centrifuge, aspiration/suction pump, patient monitors, ECG, oxygenators etc.
- I-V Diagnostic medical device: usually used for in-vitro examination of specimens.
Indian nascent industry - The domestic medical device industry imports more than 90% of sophisticated devices and thus, has an enormous scope for R&D capacity. Fortunately, the size of the medical device industry in India was USD 2.16 Billion in 2006 and it has reached USD 5.2 Billion by the year 2017.
What are the setbacks faced by medical device start-ups in India?
Yes, there are private players taking the plunge and government sector is spending big time in the healthcare sector. Still, the path to universal healthcare is challenging. Challenges faced by the businesses are mentioned below-
- Ensuring the product quality
- Regulatory compliance and government support
- Cost of product development
- Unavailability of resources
Product quality – Medical device manufacturers are proactively investing in the product development projects at a breakneck speed. The major aim of small and mid-sized manufacturers in this industry is to deliver quality in order to avoid product recalls as it directly reflects their brand name and the bottom-line.
Regulatory compliance and government support – Adhering to product safety standards and norms is mandatory. Every range of equipment – from the thermometer to imaging machines, should meet the standards in every nation where it is being sent.
Apart from this, garnering the support from the government in the form of expediting approvals, taxes and financial support, subsidized research etc. is a challenge for the budding manufacturers.
Cost of product development – The burgeoning cost of product development is one of the biggest challenges faced by start-ups in India. Technology and government regulations support the innovative minds, but the exorbitant cost is a major obstacle.
Unavailability of skilled resources, uncertain regulations, and irregular pricing environment, lack of innovation and extensive customization in the products also affect this business.
So, how can a manufacturer overcome these challenges? What is the key element that lets them grow?
You would have read so much about Enterprise Resource Planning applications. ERP has become inevitable for every organization, whether large scale or small.
Medical device industry is a Central Drugs Standard Control Organization (CDSCO) regulated environment where higher visibility, real-world benefits, and tangible ROI – are the factors responsible for its growth.Medical device ERP backs you up and allows business owners like you to stand out by beating the rest.
*Maintain strict compliance standards – CDSCO regulated compliance standards are detailed and should be followed with utmost accuracy – this directly connects with the reputation of the company. A reliable ERP software allows businesses to establish measures throughout the business processes – from procurement to shipping - while adhering to the strict regulations.
*Automation – We are living in a world where machines do the talking! Automation is a must in the error-prone areas. Advanced ERP solutions have in-built automation capabilities for vendor management, billing, execution system and so on.
*Remote access – Authorized users in an organization can access the important business information from remote locations, irrespective of the time zone.
* Cradle-to-grave traceability – The lot and serial tracking and the traceability features enable swift recall processes and audits. Managers can track and record costs and usage, predict availability and plan replenishment capacity.
Wrap up –
ERP will manage all your compliance issues, quality check of the products (from raw material to the finished good), BOMs and other aspects of the organization.






